PUBLICATIONS
From IIT Roorkee
25. Pd-Catalyzed Synthesis of Deuterated Olefins from (Hetero)Arenes and Ketones via Cross-Coupling Approach.
Happy, S.; Duklan, B.; Yamini, P.; Yadagiri, D. Chem. Commun. 2026, DOI.org/10.1039/D5CC07257C

24. Light-Driven Synthesis of 3-(Hetero)Aryl/Alkyl-1-indanone Derivatives from Indan-1,3-diones and Boronic acids via N-Tosylhydrazones.
Junaid, M.; Yamini, P.; Yadagiri, D. Tetrahedron Chem. 2025, 16, 100150 (Invited article).

23. Light-Induced Intramolecular Carbene C(sp3)–H Insertion of N-Tosylhydrazones; Synthesis of Functionalized Coumarans and Indolines.
Yamini, P.; Duklan, B.; Nirmal, M.; Yadagiri, D. Chem. Commun. 2025, 61, 17017. Among the most downloaded ChemComm publications from October 2025, both in November and October in the year 2025.

22. Convergent Synthesis of Functionalized Indolines from Alkyl Aryl Tertiary Amines and Diazoesters via Visible Light-Driven [4+1]-Annulation Approach.
Babbar, A.; Saleem, M.; Yadagiri, D. Org. Lett. 2025, 27, 8591.
Previously ChemRxiv 2025, DOI:doi.org/10.26434/chemrxiv-2025-2nh5m

21. Pd-Catalyzed Late-Stage Installation of Nitrile and Carbamoyl Groups via a Cross-Coupling Approach of Thianthrenium Salts with Isonitriles.
Happy, S.; Saleem, M.; Yadagiri, D. ACS Catalysis 2025, 15, 13401.

20. Light–Induced Reactivity of Nucleophilic Siloxycarbene with Heterocumulenes: Synthesis of ⍺-Ketoamides, Hydantoins, Oxoacetamidines, and Amides.
Saleem, M.; Abhishek, P.; Yadagiri, D. Org. Lett. 2024, 26, 10291. Highlighted on the Organic Chemistry Portal.
Check it https://www.organic-chemistry.org/abstracts/lit9/894.shtm

19. Light–Driven Intramolecular Cyclopropanation of Alkene-Tethered N-Tosylhydrazones; Synthesis of Fused-Cyclopropane 𝜸-Lactones.
Yamini, P.; Babbar, A.; Yadagiri, D. Org. Lett. 2024, 26, 6035.

18. Visible-Light Induced Siloxycarbene Addition to N=N of Azodicarboxylates: Synthesis of Acyl Hydrazides from Acylsilanes.
Saleem, M.; Ratwan, A.; Yamini, P.; Yadagiri, D. Org. Lett. 2024, 26, 2039. Highlighted on the Organic Chemistry Portal. Check it here: https://www.organic-chemistry.org/abstracts/lit9/479.shtm

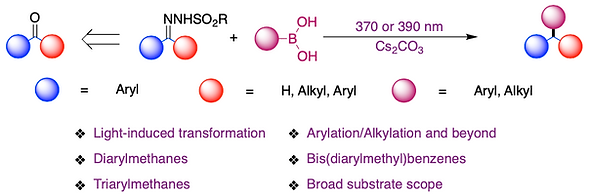
17. Light-Induced Arylation (Alkylation) of N-Sulfonylhydrazones with Boronic Acids.
Junaid, M.; Happy, S.; Yadagiri, D. Chem. Commun. 2024, 60, 2796.
16. Rhodium-Catalyzed [4+2]-Annulation of o-Acylanilines with N-Sulfonyl-1,2,3-Triazoles: Synthesis of 3-Aminoquinolines.
Rupa, K.; Yadagiri, D.; Anbarasan, P. J. Org. Chem. 2023, 88, 9077.

15. Synthesis of Dihydro-3,1-benzoxazine Derivatives from 1,3-Amino Alcohols and N-Sulfonyl-1,2,3-triazole.
Rupa, K.; Yadagiri, D.; Bagavathi, R.; Anbarasan, P. Org. Lett. 2023, 25, 3375.

Prior to IIT Roorkee
14. Denitrogenative Transformations of Pyridotriazoles and Related Compounds: Synthesis of N-Containing Heterocyclic Compounds and Beyond.
Yadagiri, D.; Rivas, M.; Gevorgyan, V. J. Org. Chem. 2020, 85, 11030.
13. Light-Induced Metal-Free Transformations of Pyridotriazoles.
Zhang, Z.†; Yadagiri, D.†; Gevorgyan, V. Chem. Sci. 2019, 10, 8399. †(These authors contributed equally to this work)
12. Transition Metal- and Light-Free Directed Amination of Remote Unactivated C(sp3)-H Bonds of Alcohols. Kurandina, D.†; Yadagiri, D.†; Rivas, M.†; Kavun, A.; Chuentragool, P.; Hayama, K.; Gevorgyan, V.
J. Am. Chem. Soc. 2019, 141, 8104. †(These authors contributed equally to this work)
11. Aliphatic Radical Relay Heck Reaction at Unactivated C(sp3)-H Sites of Alcohols. Chuentragool, P.†; Yadagiri, D.†; Morita, T.†; Sarkar, S.; Parasram, M.; Wang, Y.; Gevorgyan, V.
Angew. Chem. Int. Ed. 2019, 58, 1794. †(These authors contributed equally to this work)
This work was highlighted in Synfacts ( 2019, 15, 271).
10. Micelle-enabled Clean and Selective Sulfonylation of Polyfluoroarenes in Water under Mild Conditions.
Smith, J. D.; Ansari, T. N.; Andersson, M. P.; Yadagiri, D.; Ibrahim, F.; Liang, S.; Hammond, G. B.; Gallou, F.; Handa. S. Green. Chem. 2018, 20, 1784.
9. Rhodium-Catalyzed Synthesis of Benzopyrans via Transannulation of N-Sulfonyl-1,2,3-triazoles with 2-Hydroxybenzylalcohols.
Yadagiri, D.; Chaitanya, M.; Reddy, A. C. S.; Anbarasan, P. Org. Lett. 2018, 20, 3762.
8. Rhodium-Catalyzed Diastereoselective Synthesis of 2,2,3,3-Tetrasubstituted Indolines from N-sulfonyl-1,2,3-Triazoles and ortho-Vinyl Anilines.
Yadagiri, D.; Reddy, A. C. S.; Anbarasan, P. Chem. Sci. 2016, 7, 5934.
This work was highlighted in Synfacts (2016, 12, 823).
7. One-Pot Aminoethylation of Indoles/Pyrroles with Alkynes and Sulfonyl Azides.
Rajasekar, S.; Yadagiri, D.; Anbarasan, P. Chem. Eur. J. 2015, 21, 17079.
6. An Iodine(III) Mediated Oxidative Rearrangement of Enamines: Efficient Synthesis of α-Aminoketones. Yadagiri, D.; Anbarasan, P. Chem. Commun. 2015, 51, 14203.
5. Tandem 1,2-Sulfur Migration and (Aza)- Diels-Alder Reaction of β-Thio-α-Diazoimines: Rhodium-Catalyzed Synthesis of (Fused)- Polyhydropyridines, and Cyclohexanes.
Yadagiri, D.; Anbarasan, P. Chem. Sci. 2015, 6, 5847.
4. Recent Advances in Transition Metal Catalyzed Denitrogenative Transformation of 1,2,3-Triazoles and Related Compounds.
Anbarasan, P.; Yadagiri, D.; Rajasekar, S. Synthesis 2014, 46, 3004. (Invited review).
3. Rhodium-Catalyzed Direct Arylation of α-Diazoimines.
Yadagiri, D.; Anbarasan, P. Org. Lett. 2014, 16, 2510.
2. Rhodium-Catalyzed Cyanation of Chelation Assisted C-H Bonds.
Chaitanya, M.; Yadagiri, D.; Anbarasan, P. Org. Lett. 2013, 15, 4960.
1. Rhodium-Catalyzed Denitrogenative [2,3]-Sigmatropic Rearrangement: An Efficient Entry to Sulfur-Containing Quaternary Center.
Yadagiri, D.; Anbarasan, P. Chem. Eur. J. 2013, 19, 15115.